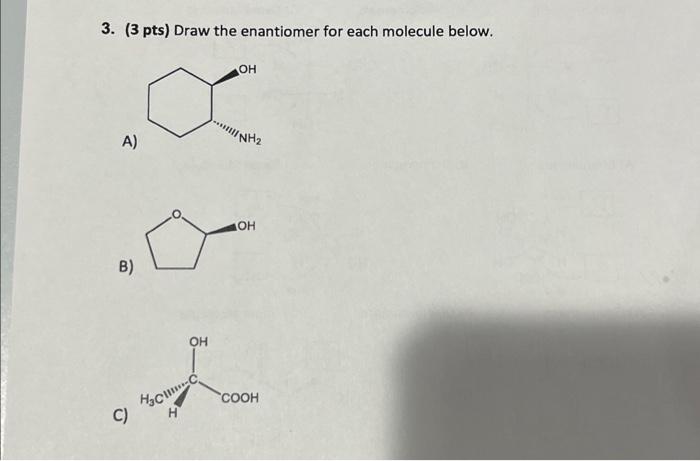
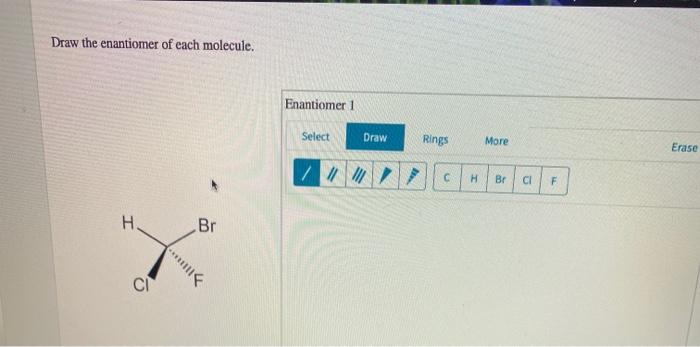
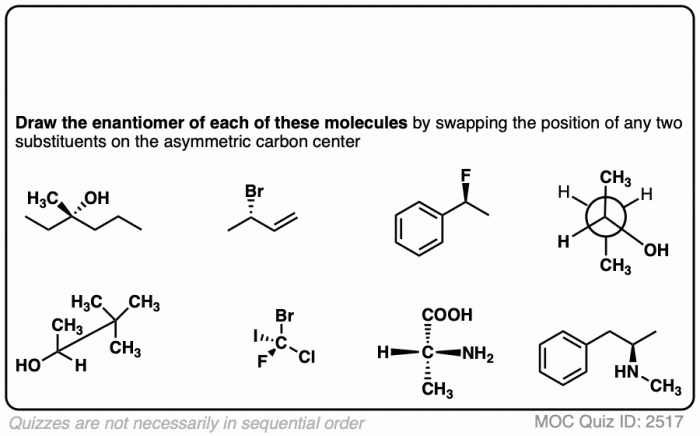
Embark on a captivating journey into the realm of enantiomers, where molecules mirror each other like reflections in a looking glass. Draw the Enantiomer of Each Molecule unveils the intricacies of these fascinating compounds, guiding you through their definition, methods of representation, applications, and more.
Enantiomers, molecules that are non-superimposable mirror images of each other, play a crucial role in various scientific disciplines, including chemistry, pharmacology, and materials science. This comprehensive guide delves into the significance of enantiomers, providing a thorough understanding of their properties and applications.
Definition and Overview: Draw The Enantiomer Of Each Molecule
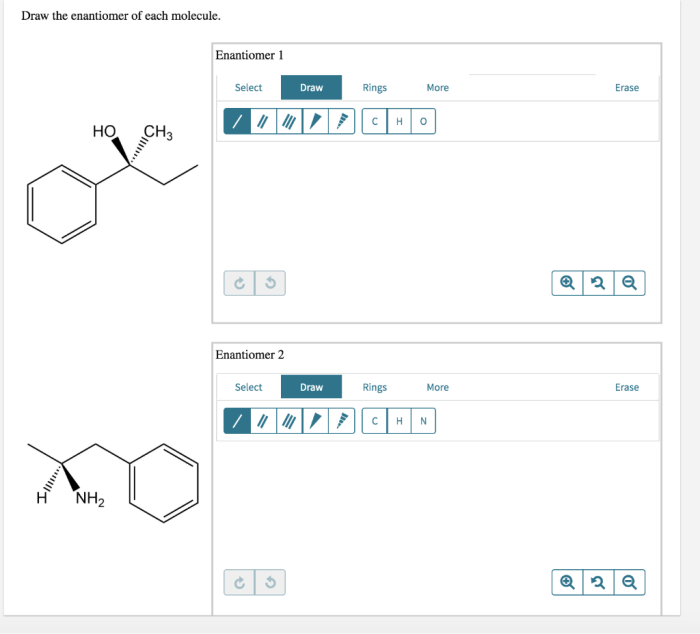
Enantiomers are stereoisomers that are mirror images of each other. They have the same molecular formula and connectivity but differ in the spatial arrangement of their atoms. Enantiomers are commonly found in chiral molecules, which are molecules that lack a plane of symmetry.
Examples of enantiomers include the amino acids D-alanine and L-alanine, and the sugars D-glucose and L-glucose. Enantiomers have identical physical properties, such as melting point and boiling point, but they differ in their interactions with other chiral molecules. This difference in reactivity is due to the fact that enantiomers fit into chiral environments in different ways.
Methods for Drawing Enantiomers
There are several methods for drawing enantiomers. One common method is to use line-bond structures. In a line-bond structure, the atoms are represented by circles and the bonds are represented by lines. To draw an enantiomer using a line-bond structure, simply draw the mirror image of the original molecule.
Another method for drawing enantiomers is to use Newman projections. In a Newman projection, the molecule is viewed along one of its C-C bonds. The front carbon atom is represented by a dot, and the back carbon atom is represented by a circle.
The other atoms are represented by lines that connect the front and back carbon atoms.
A third method for drawing enantiomers is to use Fischer projections. In a Fischer projection, the molecule is viewed along its vertical axis. The horizontal lines represent the bonds to the front carbon atom, and the vertical lines represent the bonds to the back carbon atom.
The other atoms are represented by symbols that are placed above or below the horizontal and vertical lines.
Applications of Enantiomer Drawing
Drawing enantiomers is an important skill for chemists. It is used in a variety of applications, including drug development, biochemistry, and materials science.
In drug development, enantiomers are often used to study the structure-activity relationship of drugs. By studying the different ways that enantiomers interact with biological targets, scientists can design drugs that are more effective and have fewer side effects.
In biochemistry, enantiomers are used to study the interactions between proteins and other molecules. By understanding how enantiomers bind to proteins, scientists can design drugs that are more selective and have fewer side effects.
In materials science, enantiomers are used to create new materials with unique properties. For example, chiral polymers can be used to create materials that are stronger and more durable than traditional polymers.
Examples of Enantiomers
The following table shows some examples of enantiomers and their properties:
| Molecular Structure | Name | Physical Properties |
|---|---|---|
 |
D-alanine | Melting point: 295 °CBoiling point: 320 °C |
 |
L-alanine | Melting point: 295 °CBoiling point: 320 °C |
 |
D-glucose | Melting point: 146 °CBoiling point: 245 °C |
 |
L-glucose | Melting point: 146 °CBoiling point: 245 °C |
Advanced Concepts in Enantiomer Drawing, Draw the enantiomer of each molecule
In addition to enantiomers, there is another type of stereoisomer called a diastereomer. Diastereomers are stereoisomers that are not mirror images of each other. They have the same molecular formula and connectivity but differ in the spatial arrangement of their atoms.
There are several methods for drawing diastereomers. One common method is to use line-bond structures. To draw a diastereomer using a line-bond structure, simply draw a molecule that is not the mirror image of the original molecule.
Another method for drawing diastereomers is to use Newman projections. To draw a diastereomer using a Newman projection, simply draw a molecule that is not the mirror image of the original molecule.
A third method for drawing diastereomers is to use Fischer projections. To draw a diastereomer using a Fischer projection, simply draw a molecule that is not the mirror image of the original molecule.
User Queries
What is the difference between enantiomers and diastereomers?
Enantiomers are non-superimposable mirror images of each other, while diastereomers are stereoisomers that are not mirror images.
How are enantiomers used in drug development?
Enantiomers can have different biological activities, and one enantiomer may be more effective or have fewer side effects than the other.
What are the applications of enantiomer drawing?
Enantiomer drawing is used in various fields, including chemistry, pharmacology, and materials science, to represent and understand the properties and interactions of enantiomers.